TeaM O. KUNDUZOVA / A. PARINI

Cardiometabolic remodeling : mechanisms and microenvironnement (CERAMIC)
The focus of our research program is to delineate the molecular mechanisms that govern cardiac remodeling processes and to translate these discoveries into new diagnostic and therapeutic strategies for heart failure (HF). Fibrosis, inflammation and metabolic abnormalities are major aspects of cardiac remodeling and ventricular dysfunction leading to the development and progression of HF. This is currently the central axis of our research projects that combine the expertise of clinical and academic researchers. A specific focus of our studies is to understand how metabolism regulates myocyte and stroma cell functions and how to exploit metabolic changes for therapeutic benefits to prevent adverse cardiac remodeling. We combine state-of-the-art « omics » technologies with mainstay techniques of molecular biology, cell biology, biochemistry, (patho)physiology and pharmacology to identify and characterize new actors and networks in cardiovascular biology. The translational research programs of our team foster the multidisciplinary integration of basic- and patient-oriented research, moving from bench to bedside.
TEAM

Oxana KUNDUZOVA

Angelo PARINI

Nathalie PIZZINAT

Véréna POINSOT

Jérôme RONCALLI

Charlotte ROUZAUD LABORDE

Kévin CHANDRESHWAR

Virginie JACQUES

Dimitri MARSAL

Solomiia KRAMAR

Lesia SAVCHENKO

Ryeonshi KANG

Audrey SWIADER
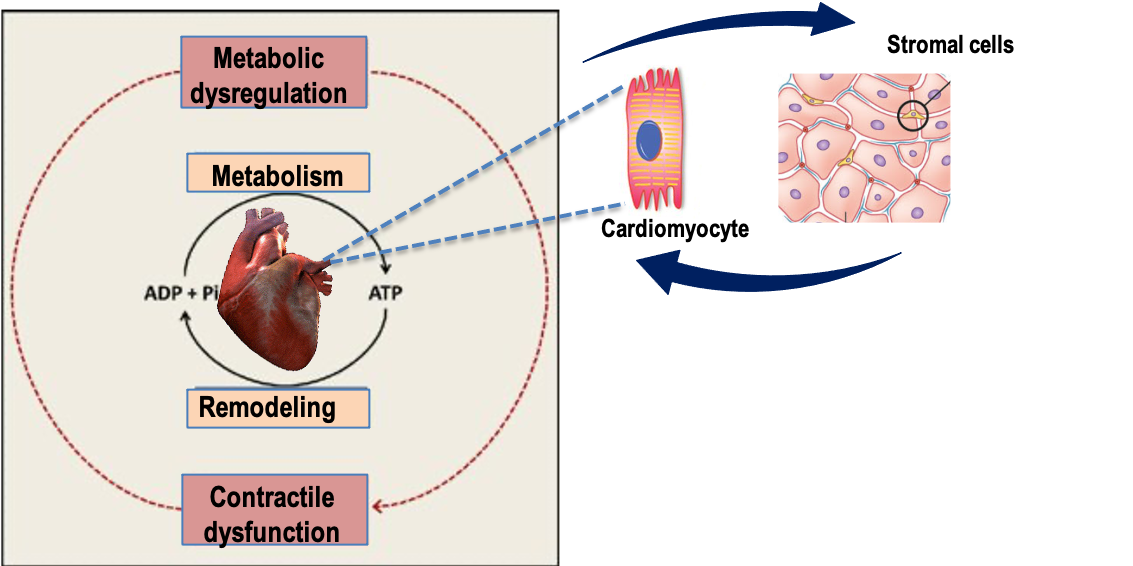
Metabolic reprogramming of stromal cells in cardiac remodeling and impact on the inflammatory microenvironment
Coordinators : O. Kunduzova, F. Savagner, V. Poinsot, D. Cussac, A. Parini
Through our research program we aim to characterize the metabolic reprogramming of fibroblast-to-myofibroblast transition in myocardial fibrotic remodeling, to define the metabolic profile of cardiac mesenchymal stromal cells in relation to their secretory activities and to establish their links with immunoinflammatory cells in HF predisposing conditions (pressure overload, obesity, aging).
Phosphoinositides metabolism in cardiac remodeling

Coordinators : F. Boal, H. Tronchère, O. Kunduzova
Our research focuses on the role of the rare phosphoinositide phosphatidylinositol 5-phosphate (PI5P) in cardiac remodeling. More specifically, we aim to decipher the involvement of its biosynthetic pathways in fibrotic and cardiometabolic remodeling. In particular, we investigate the role of the lipid kinase PIKfyve and the phosphatase myotubularin MTM1 in cardiomyocytes metabolic alterations and activation of cardiac fibroblasts during the progression of heart failure.
Translation to clinical research
Coordinators : J. Roncalli, C. Laborde, A. Parini
The translational part of our project concerns two major Axes: 1) the design of novel strategies targeting at challenges of cardiac regenerative medicine and 2) the identification of novel therapeutic targets and biomarkers of cardiac dysfunction associated with obesity and aging. Our group has a large experience on the approaches of cardiac cell therapy using bone marrow mesenchymal stem cells (BM-MSCs). In addition, we have developed an alternative strategy based on the design of bioengineered BM-MSCs/biomatrices providing the local delivery of beneficial paracrine factors without major side effects related to intramyocardial injection.
Selected publications
Apilimod alters TGFβ signaling pathway and prevents cardiac fibrotic remodeling. Cinato M, Guitou L, Saidi A, Timotin A, Sperazza E, Duparc T, Zolov SN, Giridharan SSP, Weisman LS, Martinez LO, Roncalli J, Kunduzova O, Tronchere H, Boal F. Theranostic. 2021. Pubmed
Galanin promotes autophagy and alleviates apoptosis in the hypertrophied heart through FoxO1 pathway. Martinelli I, Timotin A, Moreno-Corchado P, Marsal D, Kramar S, Loy H, Joffre C, Boal F, Tronchere H, Kunduzova O. Redox Biol. 2021. Pubmed
Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Abbadi D , Laroumanie F, Bizou M, Pozzo J, Daviaud D, Delage D, Calise D, Gaits-Iacovoni F, Dutaur M, Tortosa F, Renaud-Gabardos E , Douin-Echinard V, Prats A.C, Roncalli J, Parini A, and Pizzinat N Cardiovasc Res. 2018. Pubmed
Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy.
Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90 + subset.
FUNDINGS






Inserm/UPS UMR 1297 - I2MC Institut des Maladies Métaboliques et Cardiovasculaires
1 avenue Jean Poulhès - BP 84225 - 31432 Toulouse Cedex 4
Tél. : 05 61 32 56 00
Horaires
Du lundi au vendredi
8h30 - 12h30 / 13h45 -16h45