Team L. Martinez
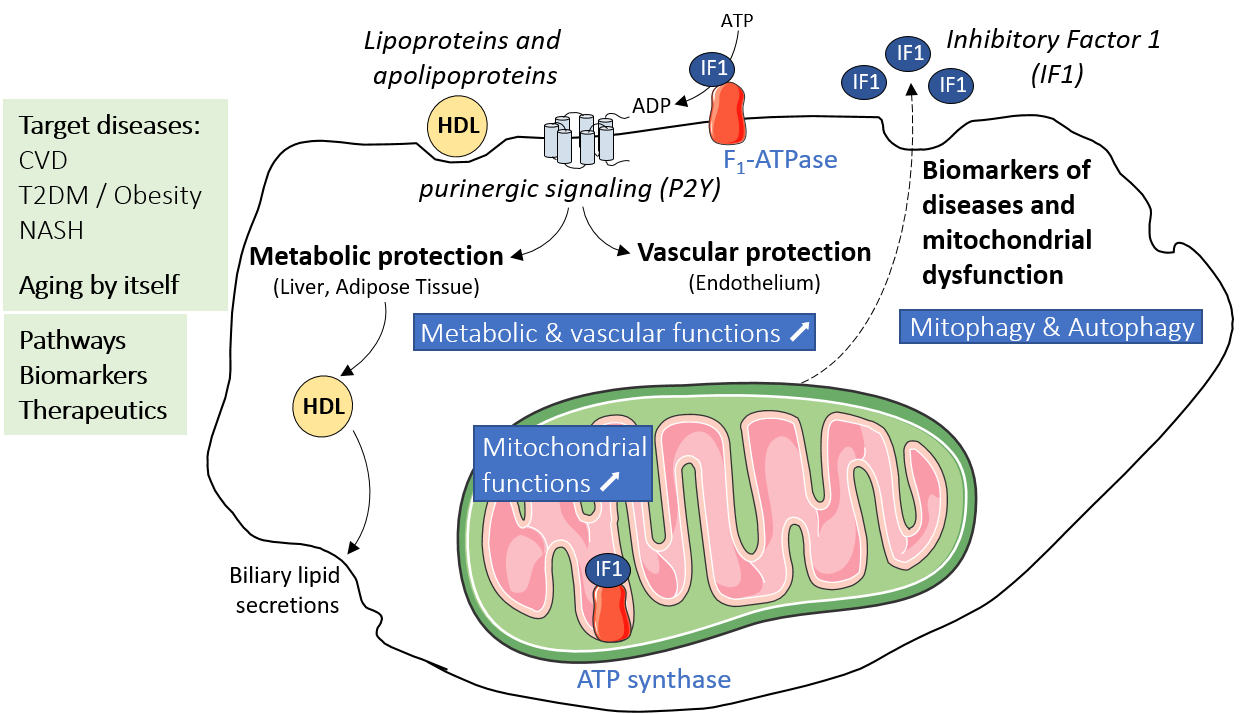
Lipoproteins and Mitochondrial adaptations in Age-related vascular & metabolic diseases (LiMitAging)
Our project aims to study the mechanism of action and the function of some players involved in lipoprotein metabolism and mitochondrial function, in different physiopathological contexts (aging, atherogenic dyslipidemia, hepatic and vascular dysfunctions).
Our objectives are to explore the physiological and pathophysiological roles of those molecular actors that regulate:
1) The oxidative phosphorylation, in particular the mitochondrial ATP synthase
2) The autophagy, in particular the mitophagy, and the involvement of this process in inter-organelle crosstalk
3) The cell surface ATP synthase and G protein-coupled P2Y receptors signaling pathways
Our research has led to the development of original drug candidates and biomarkers that are currently being validated on preclinical models (cells, organoids, animals) and human cohorts, to be used for early detection and resolution of mitochondrial dysfunctions and metabolic disorders in elderly with risk of functional decline or in population at high risk of cardiometabolic diseases.
Team members

Laurent MARTINEZ

Cendrine CABOU

Annelise GENOUX

Souad NAJIB

Cécile INGUENEAU

Bertrand PERRET

Céline VERDIER

Mohamad NASSER

Vanessa BOUGUETOCH

Guillaume COMBES

Jeanne TAWBE
High Density Lipoproteins (HDL) and ATP synthases in metabolic and cardiovascular dysfunctions

Coordinator : Laurent MARTINEZ
ATP synthase is a mitochondrial enzyme complex that we have also found expressed at the plasma membrane of several cell types as a receptor for apoA-I, the main HDL protein.
In this project, we explore the cellular functions and signaling pathways associated to the enzymatic activities of mitochondrial and cell surface ATP synthases. We are also studying the mechanisms regulating their activities. We develop original pre-clinical models and innovative pharmacological approaches to study their role in the process of aging and in different metabolic and cardiovascular dysfunctions such as dyslipidemias, atherosclerosis, diabetes and nonalcoholic steatohepatitis (Grant from Région Occitanie n°19014226 – HEPATOCARE and Région Occitanie / ERDF MP0022856 – Inspire RESPIMITAGING & MITOENERGY).

Lipoproteins and purinergic signaling in the vascular endothelium
Coordinators : Cendrine CABOU, Laurent MARTINEZ
Endothelial cells, which line the surface of blood vessels to form the endothelium, largely contribute to the homeostasis of the cardiovascular system. It has been established that a dysfunction or damage to the vascular endothelium is the cause of cardiovascular pathologies and complications.
Extracellular nucleotides such as ADP and ATP activate signaling pathways involved in endothelial function (vasomotricity, permeability, cell adhesion, etc.)
In this project, we are studying the nucleotide signaling of the vascular endothelium, in particular the one mediated by High Density Lipoprotein and involving cell surface ATP synthase, whose hydrolytic activity generates extracellular ADP which activates P2Y purinergic receptors.
In translational research, we are developing a novel family of molecules and cell therapy approaches that target nucleotide signaling to restore endothelial function (Grant from Région Occitanie / ERDF MP0018003 – THERANOVASC).

Degradative/secretory autophagy and inter-organelle crosstalk in health and disease

Coordinator : Souad NAJIB
Autophagy is an essential catabolic process to maintain cellular homeostasis program. Increasing evidences suggest a role of autophagy machinery in anabolic processes such as a plasma membrane targeting of soluble and transmembrane proteins.
In this project, we explore this versatile role of autophagy, analyse its interconnection with mitochondrial metabolism, and study the consequence of its dysfunction on the development of metabolic and cardiovascular diseases.
Biomarkers associated with lipid and energy metabolism

Coordinators : Annelise Genoux, Bertrand Perret and Laurent Martinez
This project aims to discover novel biomarkers of cardiovascular risk and metabolic syndrome, associated to mitochondrial functions or lipoprotein metabolism. Our goal is to develop diagnostic tools that will allow accurate and early risk stratification. Our work is based on a consortium comprising clinicians, epidemiologists and private companies and the use of local, national and international cohorts.
Selected publications
P2Y13 receptor deficiency favors adipose tissues lipolysis and worsens insulin resistance and fatty liver disease. Duparc T, Gore E, Combes G, Beuzelin D, Pires Da Silva J, Bouguetoch V, Marquès MA, Velazquez A, Viguerie N, Tavernier G, Arner P, Rydén M, Langin D, Sioufi N, Nasser M, Cabou C, Najib S, Martinez LO. JCI Insight. 2024. Pubmed
Plasma Level of ATPase Inhibitory Factor 1 and Intrinsic Capacity in Community-Dwelling Older Adults: Prospective Data From the MAPT Study. da Silva JA, Martinez LO, Rolland Y, Najib S, Croyal M, Perret B, Jabrane-Ferrat N, El Costa H, Guyonnet S, Vellas B, de Souto Barreto P; MAPT/DSA group. J Gerontol A Biol Sci Med Sci. 2024. Pubmed
Associations between physical activity levels and ATPase inhibitory factor 1 concentrations in older adults. Raffin J, Rolland Y, Genoux A, Combes G, Croyal M, Perret B, Guyonnet S, Vellas B, Martinez LO, de Souto Barreto P; MAPT/DSA Group. J Sport Health Sci. 2023. Pubmed
Intermittent Fasting Resolves Dyslipidemia and Atherogenesis in Apolipoprotein E-Deficient Mice in a Diet-Dependent Manner, Irrespective of Sex. Mérian J, Ghezali L, Trenteseaux C, Duparc T, Beuzelin D, Bouguetoch V, Combes G, Sioufi N, Martinez LO, Najib S. Cells. 2023. Pubmed
Plasma level of ATPase inhibitory factor 1 (IF1) is associated with type 2 diabetes risk in humans: A prospective cohort study. Pires Da Silva J, Wargny M, Raffin J, Croyal M, Duparc T, Combes G, Genoux A, Perret B, Vellas B, Guyonnet S, Thalamas C, Langin D, Moro C, Viguerie N, Rolland Y, Barreto PS, Cariou B, Martinez LO; MAPT/DSA Group. Diabetes Metab. 2023. Pubmed
ILS NOUS SOUTIENNENT

Feder

ANR

fondation de france

region occitanie




Inserm/UPS UMR 1297 - I2MC Institut des Maladies Métaboliques et Cardiovasculaires
1 avenue Jean Poulhès - BP 84225 - 31432 Toulouse Cedex 4
Tél. : 05 61 32 56 00
Horaires
Du lundi au vendredi
8h30 - 12h30 / 13h45 -16h45