Team C. Galés/JM. Sénard
Molecular and clinical determinants of cardiac architecture (ARCHI-CARD)
The physiologic response of an organ relies on a complex interplay between the different cell types structuring the tissue. At the cellular level, the response arises from the plasma membrane through different receptors, channels, pumps… that integrate and proceed the extracellular stimuli (chemical, mechanical). Alteration of the plasma membrane response is a hallmark of number of patho-physiological situations. Now, how the plasma membrane and the overall cell architecture behave in such pathologies, what is the impact on the surface organization and functions of the proteins inserted in the plasma membrane and more largely what is the impact on the neighboring cells still remain poorly understood.
In this context, the research program of our team focuses on understanding the clinical/molecular determinants of the cardiac architecture/function.
Our team is highly interdisciplinary integrating people from basic and medical research in pharmacology, cellular biology, cardiology and neurology. As a consequence, our approach also relies on a high interdisciplinarity starting from the identification of the molecular/cellular mechanisms to the integration in animal models but also in humans thanks to our collaboration with the Toulouse CHU hospital.
Team members

Céline GALÉS

Jean-Michel SÉNARD

Céline GUILBEAU-FRUGIER

Clément KARSENTY

Véronique PONS

Thomas FARGE

Yannis SAINTE-MARIE

Frédérique SAVAGNER

Daniel CUSSAC

Nicolas PATALUCH

Amandine WAHART

Sabrina BENAOUADI

Emma DUPONT

Emma BERNARD

Fabien DESPAS

Jean-Philippe MAURY

Maxime BENEYTO

Yoann ZELMAT

Varravaddheay ONG-MEANG
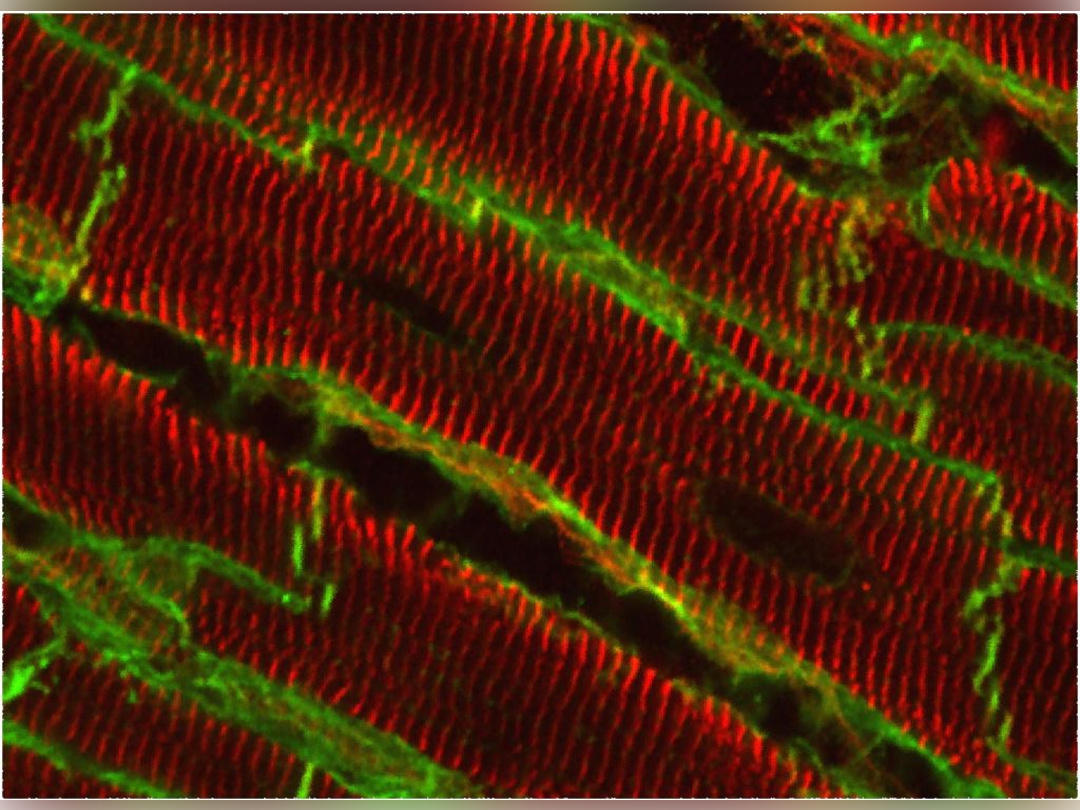
Architecture of the lateral membrane of cardiomyocytes
Coordinators : Céline Galés & Jean-Michel Sénard
Defects in cardiomyocyte (CM) signaling are a hallmark of heart failure (HF) from all origins. However, most of the studies have focused on the end-stage pathology. Whether these modifications are causes or consequences still remain to be answered and are essential for the development of new efficient therapeutics. However, the intracellular signaling outcome of a cell is tightly dependent on its origin, i.e. the cell surface. How the architecture of the CM surface is temporally modified upon cardiac stresses and how this can impact the 3D heart structure, the CM response and the overall heart function remain fundamental questions in cardiology.
Thanks to high resolution microscopies (AFM, MET) we optimized during these last years, we previously reported and accurately characterized in the cardiac tissue the architectural organization of the lateral membrane of adult CMs with periodic crests related to the presence of subsarcolemmal mitochondria (SSM) whose role is unknown and that are rapidly lost after myocardial infarction before T-Tubule disorganization, a common HF hallmark. Our goal is now to better understand the crest loss: i/ is it specific or generic to all HF origins? ii/ what is the trigger? iii/ what are the crest determinants/ modulators?, iv/ how to prevent the crest loss?
G protein coupled receptor (GPCR) organization at the cell surface and its role in ligand efficacy

Coordinators : Véronique Pons & Céline Galés
GPCRs represent the major pharmaceutical drug target. However, many drugs are associated with harmful side-effects and a poor clinical benefit-risk ratio that limit their use.
Recently, the concept of biased-agonism (ligand selecting some receptor-signaling pathways) paved the way for the development of pathway-specific drugs with higher efficacy and lower adverse events. Now, understanding the link between GPCR at the cell surface and the signaling/physiological outcome remains a major challenge for a more rational design of biased ligands.
By multiplexing high-resolution tools (FRET/BRET probes) to dissect and image the spatiotemporal GPCR signaling in living cells and 3D tissues, our goal is to identify the molecular basis underlying biased agonism/ligand efficacy at multiple cardiovascular GPCR drug targets (AT1-R, b2-AR, P2Y-R) by taking into account the existence of multiple receptor populations and different membrane architectures at the cell surface.
Selected publications
Cardiac sensory afferents modulate susceptibility to anxio-depressive behaviour in a mouse model of chronic heart failure. KERMORGANT M, BEN SALEM J, IACOVONI J, CALISE D, DAHAN L, GUIARD BP, LOPEZ S, LAIREZ O, LASBORIES A, NASR N, PAVY-LE TRAON A, BEAUDRY F, SENARD JM, ARVANITIS DN. Acta Physiol (Oxf). 2021 Apr;231(4):e13601. doi: 10.1111/apha.13601. PMID: 33316126
Deciphering biased inverse agonism of cangrelor and ticagrelor at P2Y12 receptor. GARCIA C, MAUREL-RIBES A, NAUZE M, N’GUYEN D, MARTINEZ LO, PAYRASTRE B, SENARD JM, GALES C, PONS V. Cell Mol Life Sci. 2019 Feb;76(3):561-576. doi: 10.1007/s00018-018-2960-3. PMID: 30406277
Structural evidence for a new elaborate 3D-organization of the cardiomyocyte lateral membrane in adult mammalian cardiac tissues.GUILBEAU-FRUGIER C, CAUQUIL M, KARSENTY C, LAIREZ O , DAMBRIN C, PAYRE B, CASSARD H, JOSSE C, SEGUELAS MH, ALLART S,BRANCHEREAU M, HEYMES C, MANDEL F, DELISLE MB, PATHAK A, DAGUE E, SENARD JM, GALES C. Cardiovasc Res. 2018 Oct 17. doi: 10.1093/cvr/cvy256. PMID: 30329023
Cardioprotective Angiotensin-(1-7) Peptide Acts as a Natural-Biased Ligand at the Angiotensin II Type 1 Receptor GALANDRIN S, DENIS C, BOULARAN C, MARIE J, M’KADMI C, PILETTE C, DUBROCA C, NICAISE Y, SEGUELAS MH, N’GUYEN D, BANÈRES JL, PATHAK A, SÉNARD JM, GALÉS C. Hypertension. 2016 Dec;68(6):1365-1374. doi: 10.1161/HYPERTENSIONAHA.116.08118. PMID: 27698068
Dual agonist occupancy of AT1-R-α2C-AR heterodimers results in atypical Gs-PKA signaling. BELLOT M, GALANDRIN S, BOULARAN C, MATTHIES HJ, DESPAS F, DENIS C, JAVITCH J, MAZÈRES S, SANNI SJ, PONS V, SEGUELAS MH, HANSEN JL, PATHAK A, GALLI A, SÉNARD JM, GALÉS C. Nat Chem Biol. 2015 Apr;11(4):271-9. doi: 10.1038/nchembio.1766. PMID: 25706338
FUNDINGS

CNES

FFC

Indorsia

FRM

fondation bettencourt schueller

fondation d efrance

hopitaux de toulouse


Inserm/UPS UMR 1297 - I2MC Institut des Maladies Métaboliques et Cardiovasculaires
1 avenue Jean Poulhès - BP 84225 - 31432 Toulouse Cedex 4
Tél. : 05 61 32 56 00
Horaires
Du lundi au vendredi
8h30 - 12h30 / 13h45 -16h45