équipe C.Galés / JM. Sénard
Déterminants moléculaires et cliniques de l’architecture cardiaque (ARCHI-CARD)
La fonction physiologique d’un organe repose sur une interaction complexe entre les différents types cellulaires qui structurent le tissu. Au niveau cellulaire, le signal débute à la membrane plasmique à travers différents récepteurs, canaux, pompes… qui intègrent et convertissent les stimuli extracellulaires (chimiques, mécaniques). L’altération de la signalisation au niveau de la membrane plasmique est une caractéristique commune à un grand nombre de conditions pathologiques. Il reste désormais à comprendre : i/ comment la membrane plasmique et l’architecture cellulaire globale se comportent dans ces pathologies ? ii/ quel est l’impact sur l’organisation de surface et sur les fonctions des protéines insérées dans la membrane plasmique ? iii/ quel est l’impact sur les cellules voisines ?
Dans ce contexte, le programme de recherche de notre équipe vise à comprendre les déterminants moléculaires et cliniques de l’architecture et de la fonction cardiaques. Notre équipe est hautement interdisciplinaire et intègre des personnes issues de la recherche fondamentale et médicale dans le domaine de la pharmacologie, de la biologie cellulaire, de la cardiologie et de la neurologie. En conséquence, notre approche repose également sur une forte interdisciplinarité allant de l’identification des mécanismes moléculaires/cellulaires à l’intégration dans des modèles animaux mais aussi chez l’Homme grâce à notre collaboration avec l’hôpital CHU de Toulouse.
L’Équipe

Céline GALÉS

Jean-Michel SÉNARD

Céline GUILBEAU-FRUGIER

Clément KARSENTY

Véronique PONS

Thomas FARGE

Yannis SAINTE-MARIE

Frédérique SAVAGNER

Daniel CUSSAC

Nicolas PATALUCH

Amandine WAHART

Sabrina BENAOUADI

Emma DUPONT

Emma BERNARD

Fabien DESPAS

Jean-Philippe MAURY

Maxime BENEYTO

Yoann ZELMAT

Varravaddheay ONG-MEANG
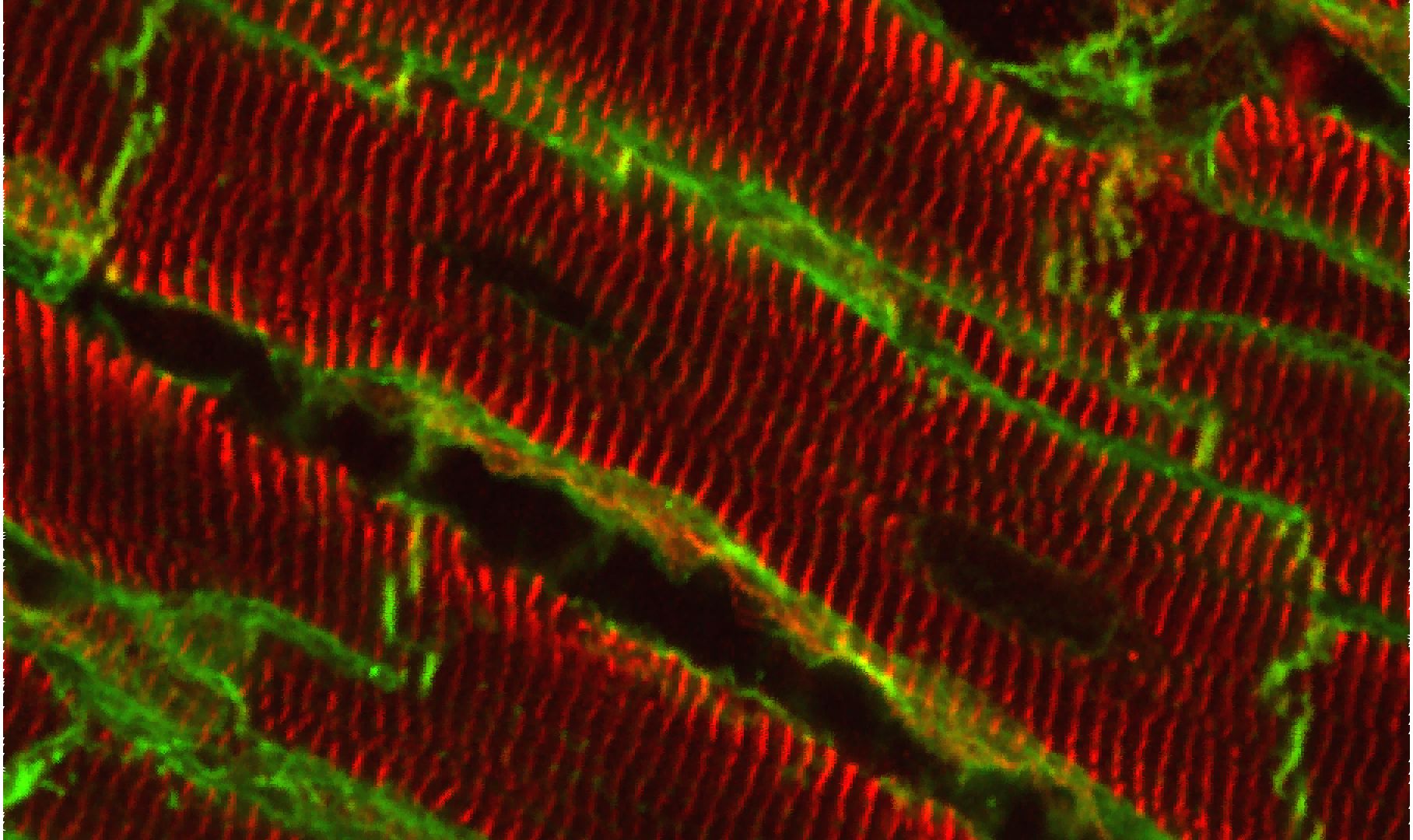
Architecture of the lateral membrane of cardiomyocytes
Coordinateurs : Céline Galés & Jean-Michel Sénard
Defects in cardiomyocyte (CM) signaling are a hallmark of heart failure (HF) from all origins. However, most of the studies have focused on the end-stage pathology. Whether these modifications are causes or consequences still remain to be answered and are essential for the development of new efficient therapeutics. However, the intracellular signaling outcome of a cell is tightly dependent on its origin, i.e. the cell surface. How the architecture of the CM surface is temporally modified upon cardiac stresses and how this can impact the 3D heart structure, the CM response and the overall heart function remain fundamental questions in cardiology.
Thanks to high resolution microscopies (AFM, MET) we optimized during these last years, we previously reported and accurately characterized in the cardiac tissue the architectural organization of the lateral membrane of adult CMs with periodic crests related to the presence of subsarcolemmal mitochondria (SSM) whose role is unknown and that are rapidly lost after myocardial infarction before T-Tubule disorganization, a common HF hallmark. Our goal is now to better understand the crest loss: i/ is it specific or generic to all HF origins? ii/ what is the trigger? iii/ what are the crest determinants/ modulators?, iv/ how to prevent the crest loss?
G protein coupled receptor (GPCR) organization at the cell surface and its role in ligand efficacy

Coordinatrices : Véronique Pons & Céline Galés
GPCRs represent the major pharmaceutical drug target. However, many drugs are associated with harmful side-effects and a poor clinical benefit-risk ratio that limit their use.
Recently, the concept of biased-agonism (ligand selecting some receptor-signaling pathways) paved the way for the development of pathway-specific drugs with higher efficacy and lower adverse events. Now, understanding the link between GPCR at the cell surface and the signaling/physiological outcome remains a major challenge for a more rational design of biased ligands.
By multiplexing high-resolution tools (FRET/BRET probes) to dissect and image the spatiotemporal GPCR signaling in living cells and 3D tissues, our goal is to identify the molecular basis underlying biased agonism/ligand efficacy at multiple cardiovascular GPCR drug targets (AT1-R, b2-AR, P2Y-R) by taking into account the existence of multiple receptor populations and different membrane architectures at the cell surface.
sélection de publications
Atomic force microscopy-single-molecule force spectroscopy unveils GPCR cell surface architecture.
DAGUE E*, PONS V*, ROLAND A, AZAÏS JM, ARCUCCI S, LACHAIZE V, VELMONT S, TREVISIOL E, N’GUYEN D, SÉNARD JM, GALÉS C. Commun Biol. 2022 Mar 10;5(1):221. doi: 10.1038/s42003-022-03162-w. (*co first authors). Pubmed
Cardiac sensory afferents modulate susceptibility to anxio-depressive behaviour in a mouse model of chronic heart failure. KERMORGANT M, BEN SALEM J, IACOVONI J, CALISE D, DAHAN L, GUIARD BP, LOPEZ S, LAIREZ O, LASBORIES A, NASR N, PAVY-LE TRAON A, BEAUDRY F, SENARD JM, ARVANITIS DN. Acta Physiol (Oxf). 2021 Apr;231(4):e13601. doi: 10.1111/apha.13601. Pubmed
Deciphering biased inverse agonism of cangrelor and ticagrelor at P2Y12 receptor. GARCIA C, MAUREL-RIBES A, NAUZE M, N’GUYEN D, MARTINEZ LO, PAYRASTRE B, SENARD JM, GALES C, PONS V. Cell Mol Life Sci. 2019 Feb;76(3):561-576. doi: 10.1007/s00018-018-2960-3. Pubmed
Structural evidence for a new elaborate 3D-organization of the cardiomyocyte lateral membrane in adult mammalian cardiac tissues.GUILBEAU-FRUGIER C, CAUQUIL M, KARSENTY C, LAIREZ O , DAMBRIN C, PAYRE B, CASSARD H, JOSSE C, SEGUELAS MH, ALLART S,BRANCHEREAU M, HEYMES C, MANDEL F, DELISLE MB, PATHAK A, DAGUE E, SENARD JM, GALES C. Cardiovasc Res. 2018 Oct 17. doi: 10.1093/cvr/cvy256. Pubmed
Cardioprotective Angiotensin-(1-7) Peptide Acts as a Natural-Biased Ligand at the Angiotensin II Type 1 Receptor GALANDRIN S, DENIS C, BOULARAN C, MARIE J, M’KADMI C, PILETTE C, DUBROCA C, NICAISE Y, SEGUELAS MH, N’GUYEN D, BANÈRES JL, PATHAK A, SÉNARD JM, GALÉS C. Hypertension. 2016 Dec;68(6):1365-1374. doi: 10.1161/HYPERTENSIONAHA.116.08118. Pubmed
ILS NOUS SOUTIENNENT

CNES

FFC

Indorsia

FRM

fondation bettencourt schueller

fondation d efrance

hopitaux de toulouse


Inserm/UPS UMR 1297 - I2MC Institut des Maladies Métaboliques et Cardiovasculaires
1 avenue Jean Poulhès - BP 84225 - 31432 Toulouse Cedex 4
Tél. : 05 61 32 56 00
Horaires
Du lundi au vendredi
8h30 - 12h30 / 13h45 -16h45