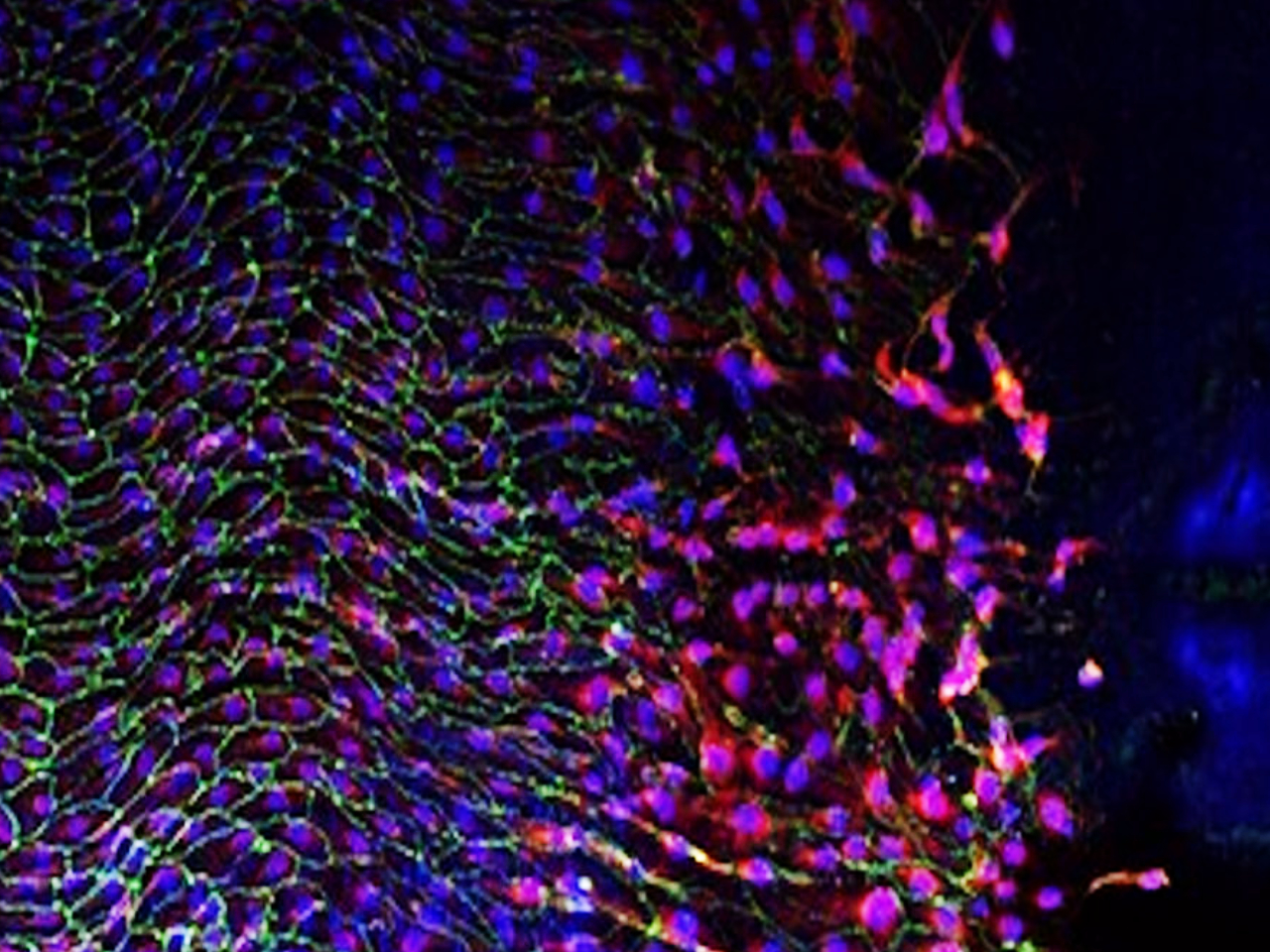
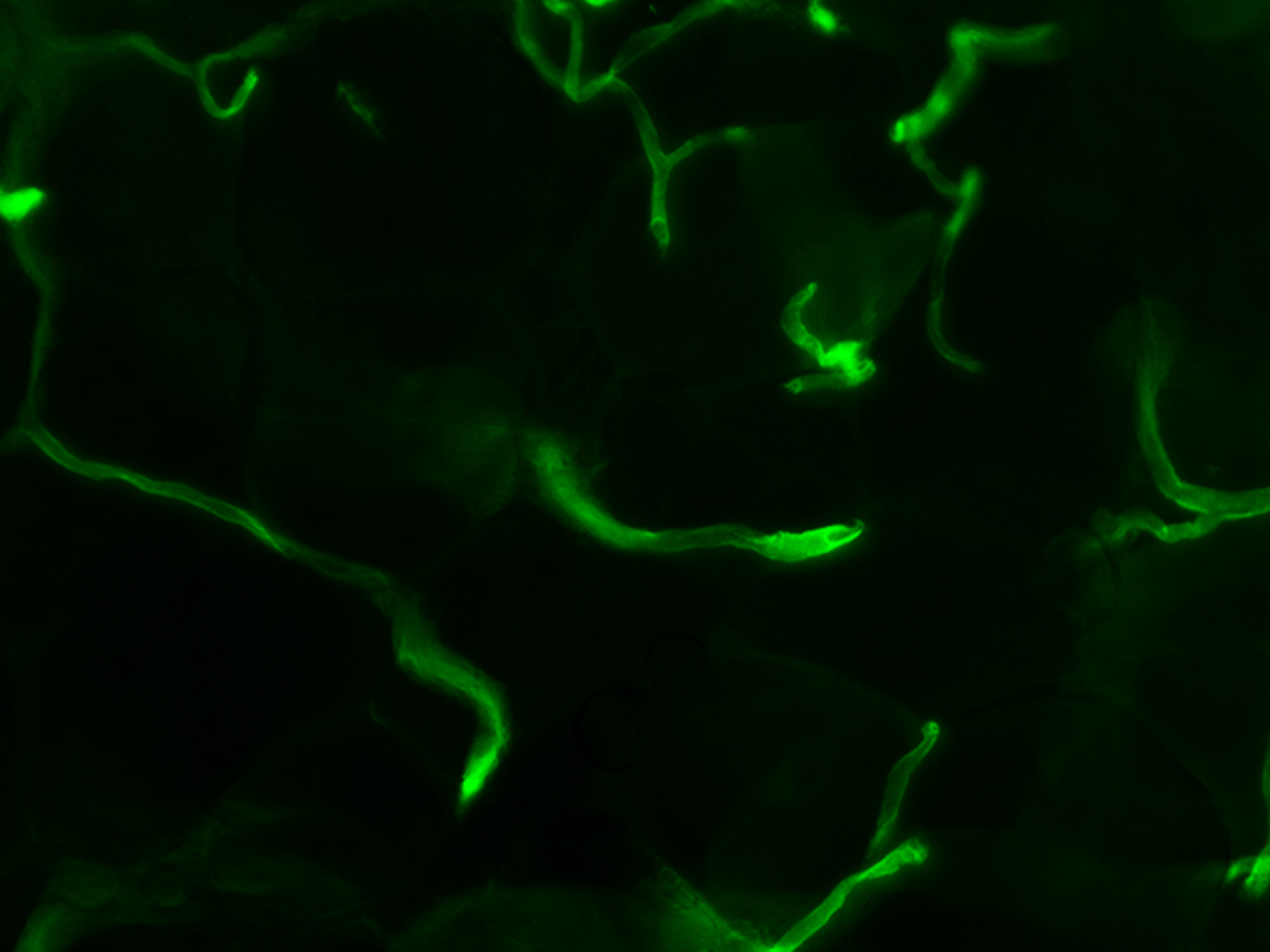
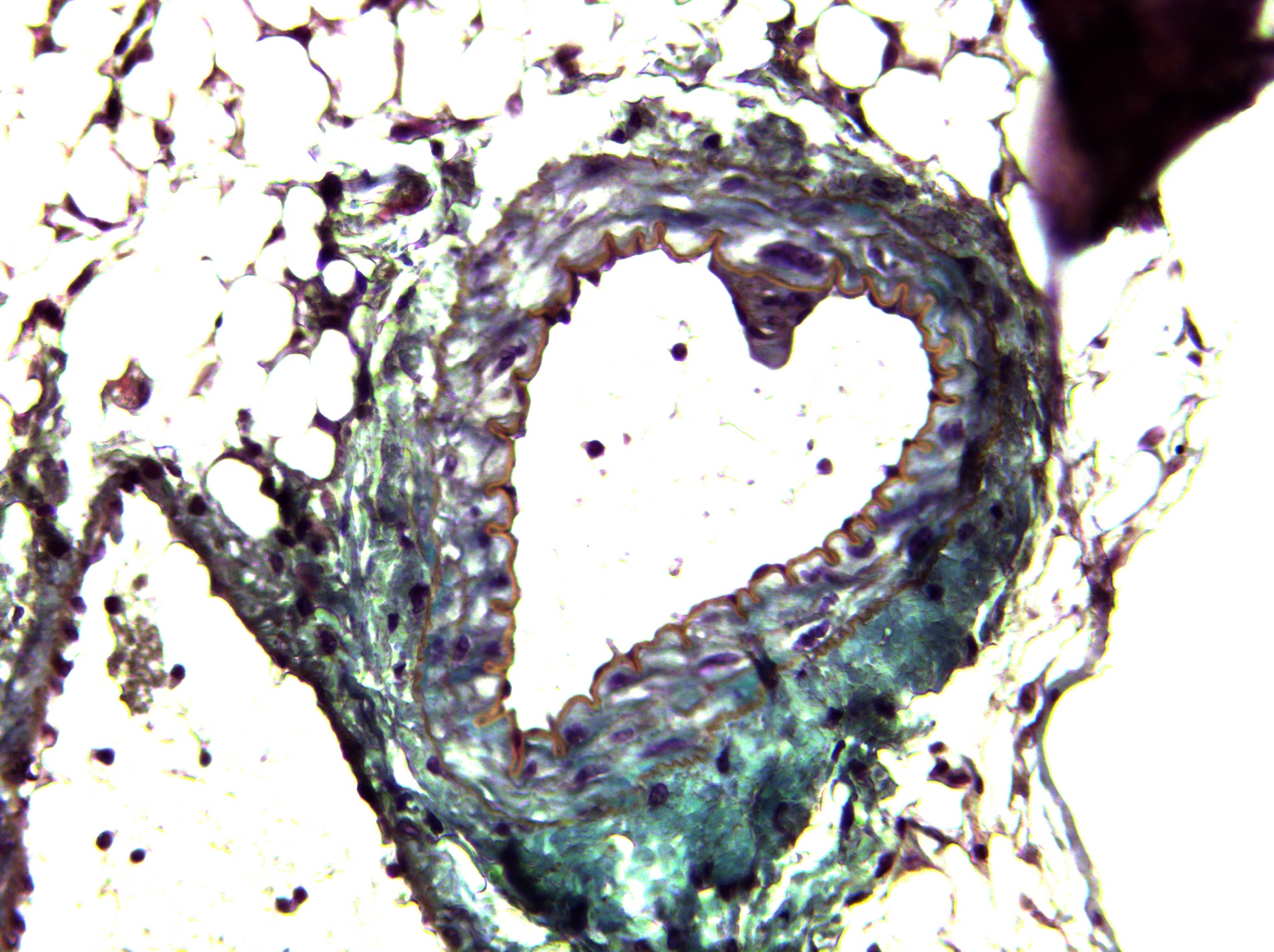
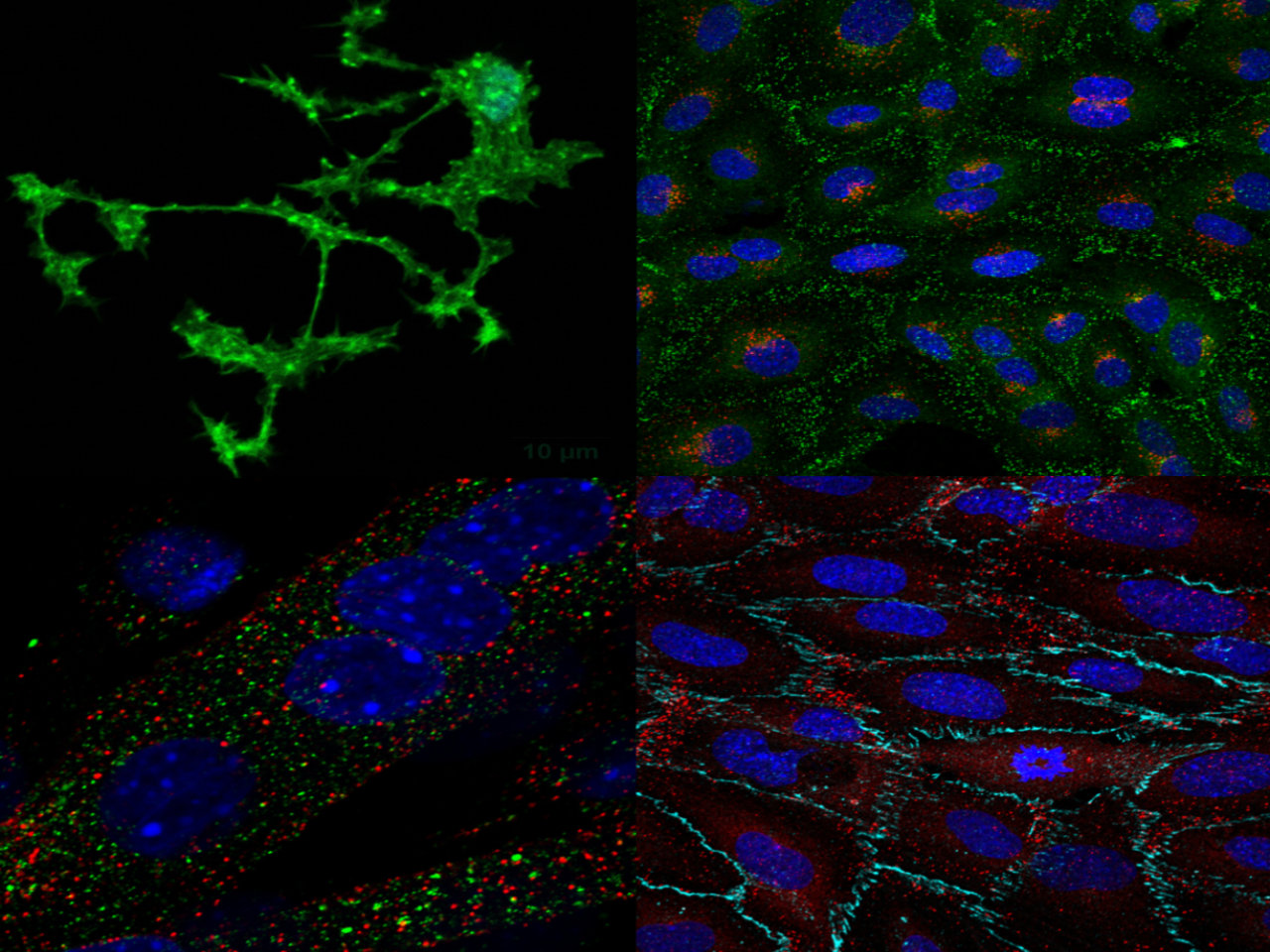
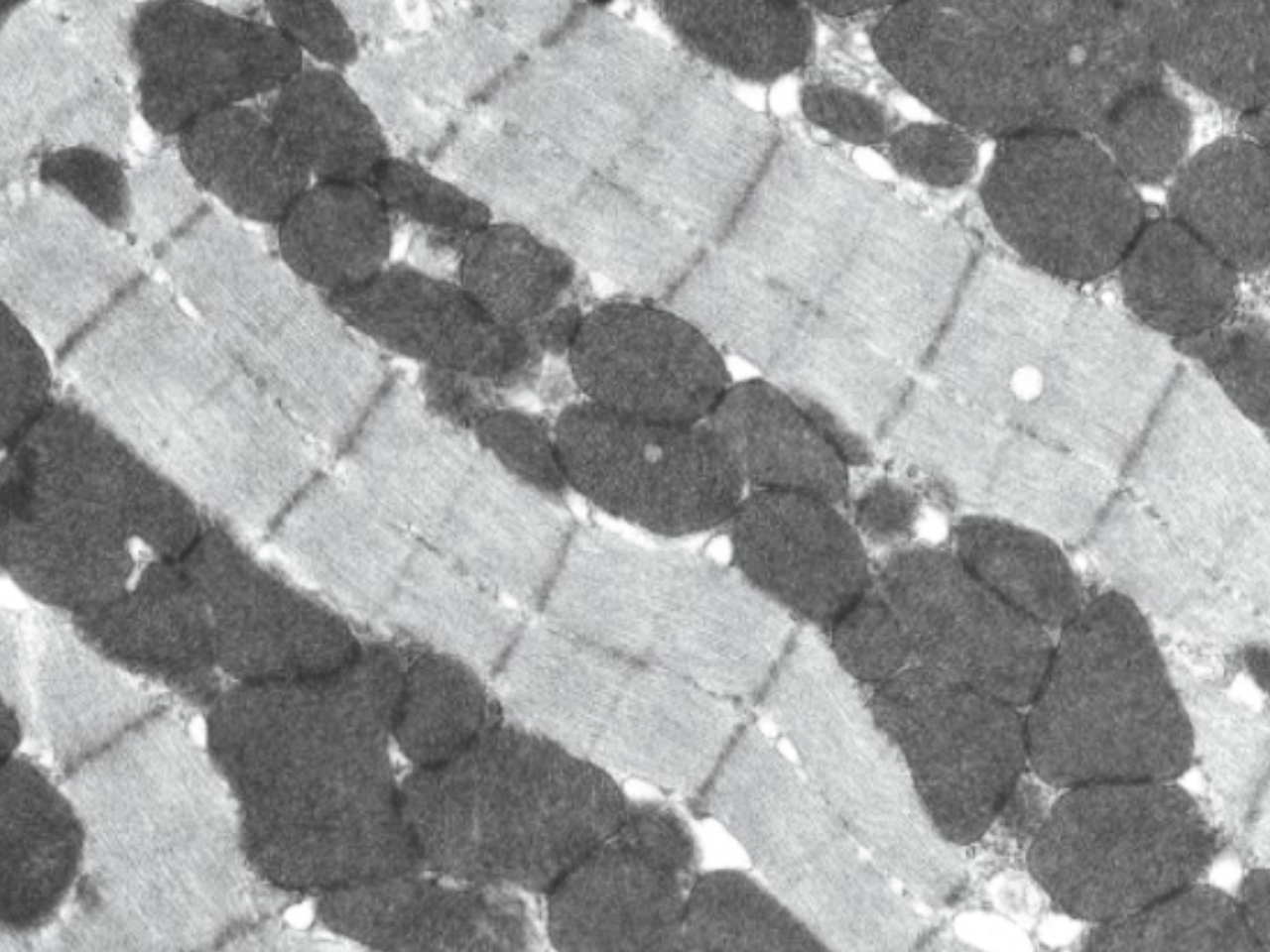
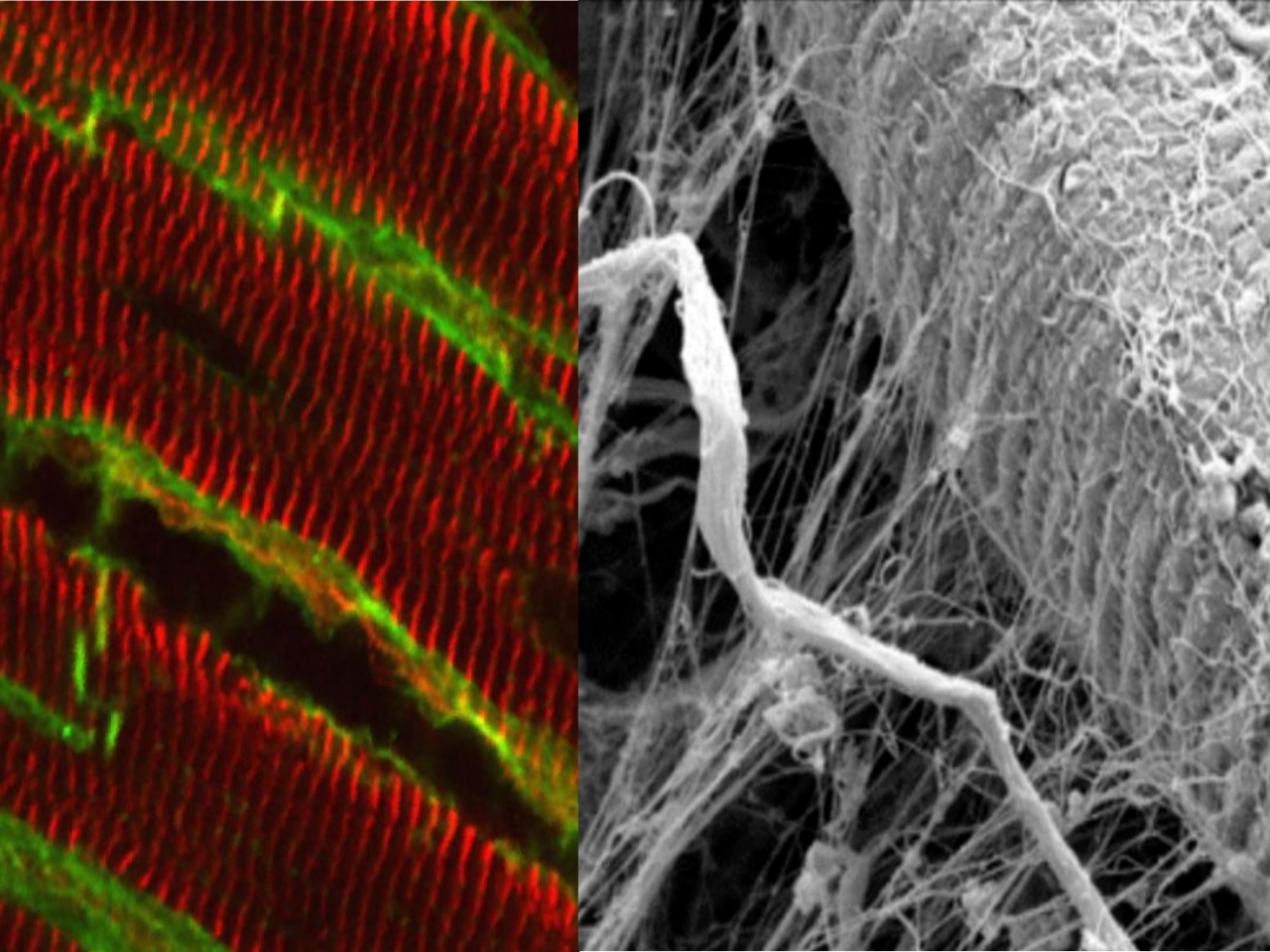
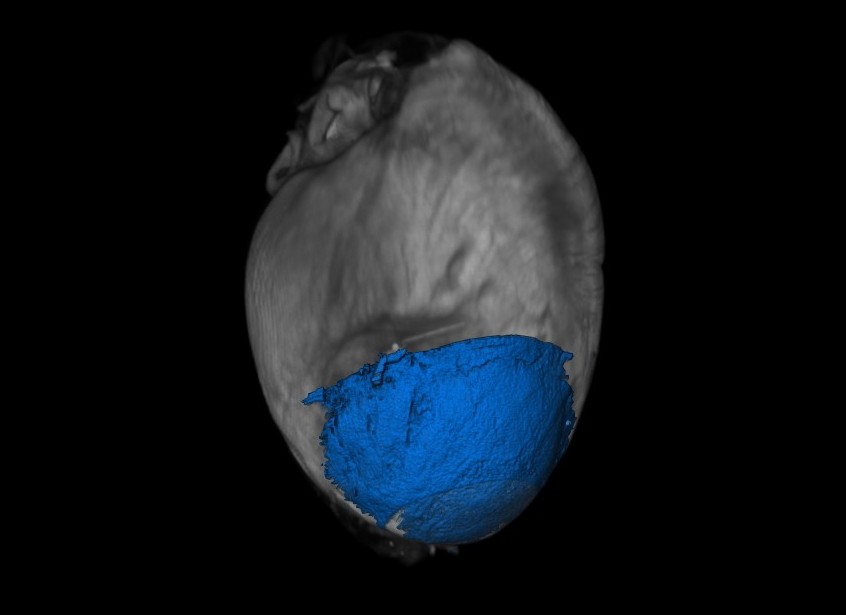
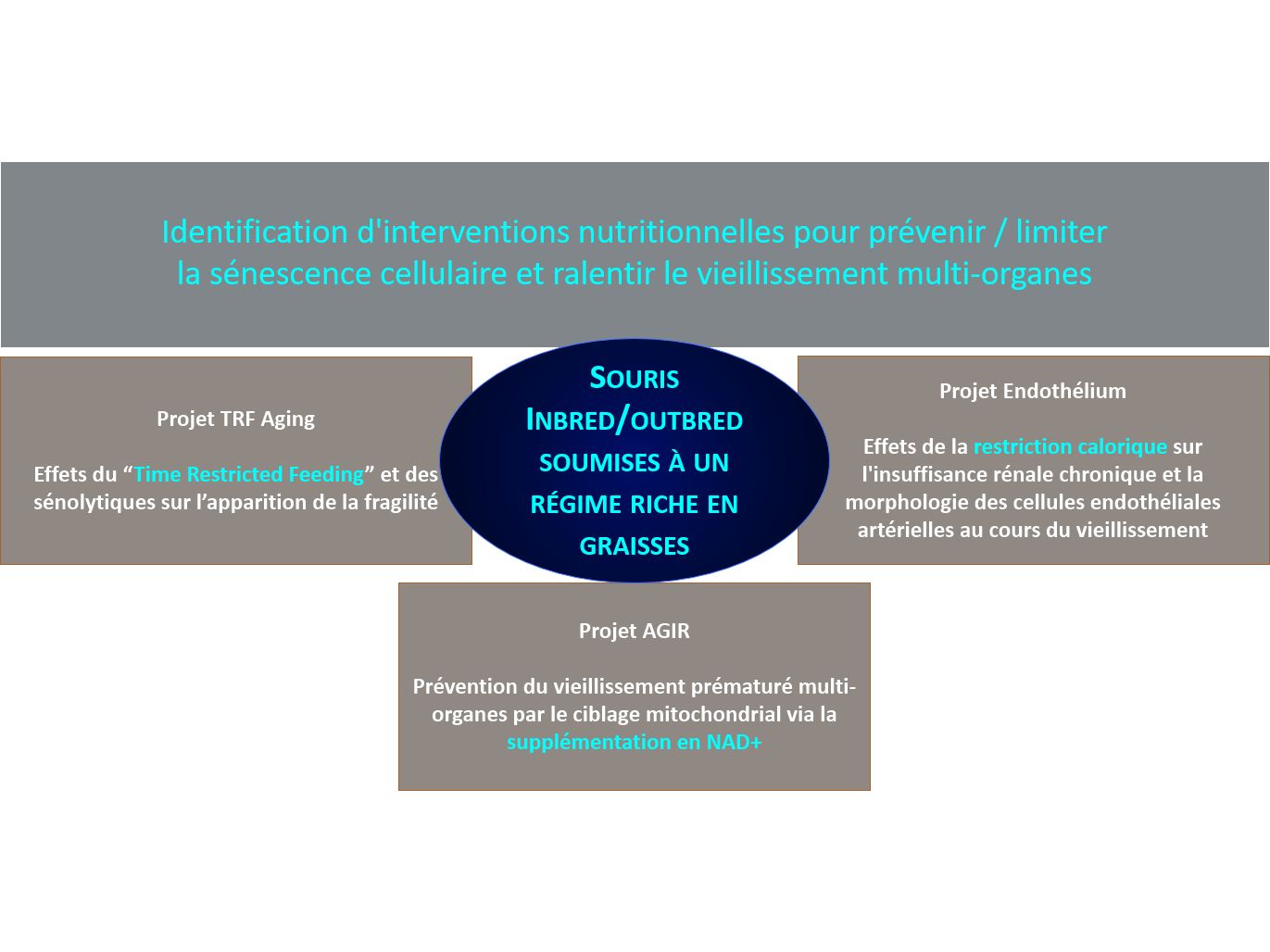
Research overview at the I2MC
METABOLIC DISEASES
Obesity, diabetes, dyslipidemia, fatty liver disease, intestinal dysbiosis
CARDIOVASCULAR AND KIDNEY DISEASES
Vessel diseases, atherosclerosis, thrombosis, heart and kidney failures
The 15 i2mc teams
Laurent Martinez
Lipoproteins and Mitochondrial adaptations in Age-related vascular & metabolic diseases (LiMitAging)
Françoise Lenfant & Coralie Fontaine
ESTrogEn Receptor Modulation to prevent aging-related metabolic and vascular dysfunctions (ESTER)
Bernard Payrastre & Sonia Séverin
Lipidomics And Signaling In Platelet Production, Thrombosis And Cell Homeostasis
Oxana Kunduzova & Angelo Parini
Cardiometabolic remodeling: mechanisms and microenvironment (CERAMIC)
Céline Galés & Jean-Michel Sénard
Molecular and clinical determinants of cardiac architecture (ARCHI-CARD)
The research programs at i2mc
Cross-cutting programs
European Programs
Research Integrity

Scientific integrity and its associated issues concern all areas of research: the research itself, the dissemination of knowledge, the training of students, evaluations and expert appraisals. Should you have any questions, require advice or wish to report misconduct, please contact Inserm’s Scientific Integrity Office.
In addition Nathalie NASR is identified as Scientific Integrity Referent (RIS) at the I2MC.
All staff registered with the Institute’s profile are, whatever their status, required to comply with the national charter of ethics for research professions which commits all French research operators and describes the principles of a rigorous scientific approach and integrity, in accordance with international and European reference texts. It also helps meet publishers’ integrity requirements.
publications


Inserm/UPS UMR 1297 - I2MC Institut des Maladies Métaboliques et Cardiovasculaires
1 avenue Jean Poulhès - BP 84225 - 31432 Toulouse Cedex 4
Tél. : 05 61 32 56 00
Horaires
Du lundi au vendredi
8h30 - 12h30 / 13h45 -16h45