team A. Bouloumié
-DINAMIX: Adipose tissues and vasculo-metabolic flexibility
Our research aims to elucidate the molecular and cellular mechanisms involved in metabolic adaptation in physiological contexts and during aging, whether physiological or accelerated by obesity.
We focus on 1) characterizing the cellular and functional heterogeneity of different white and brown adipose tissue deposits, and 2) determining their contribution to vasculometabolic flexibility. At the same time, we are studying the influence of the body distribution of adipose tissue on alterations in the regeneration and barrier function of the intestinal epithelium present in chronic inflammatory bowel diseases and in contexts of obesity.
L’Équipe

Anne BOULOUMIE-DIEHL

Anaïs BRIOT

Xavier COLLET

Audrey FERRAND

Florence TATIN

Pauline DECAUNES BESSEDE

Léopold Devineaux

Muriel QUARANTA-NICAISE

Juline MARJOLLET

Alexis ARCAS

Anne Gosset

Duvan ROJAS-GARCIA

Théo CALDERON

Rémi CENTRES
Metabolic function of microvascular endothelium: Heterogeneity, nutrient sensing and handling
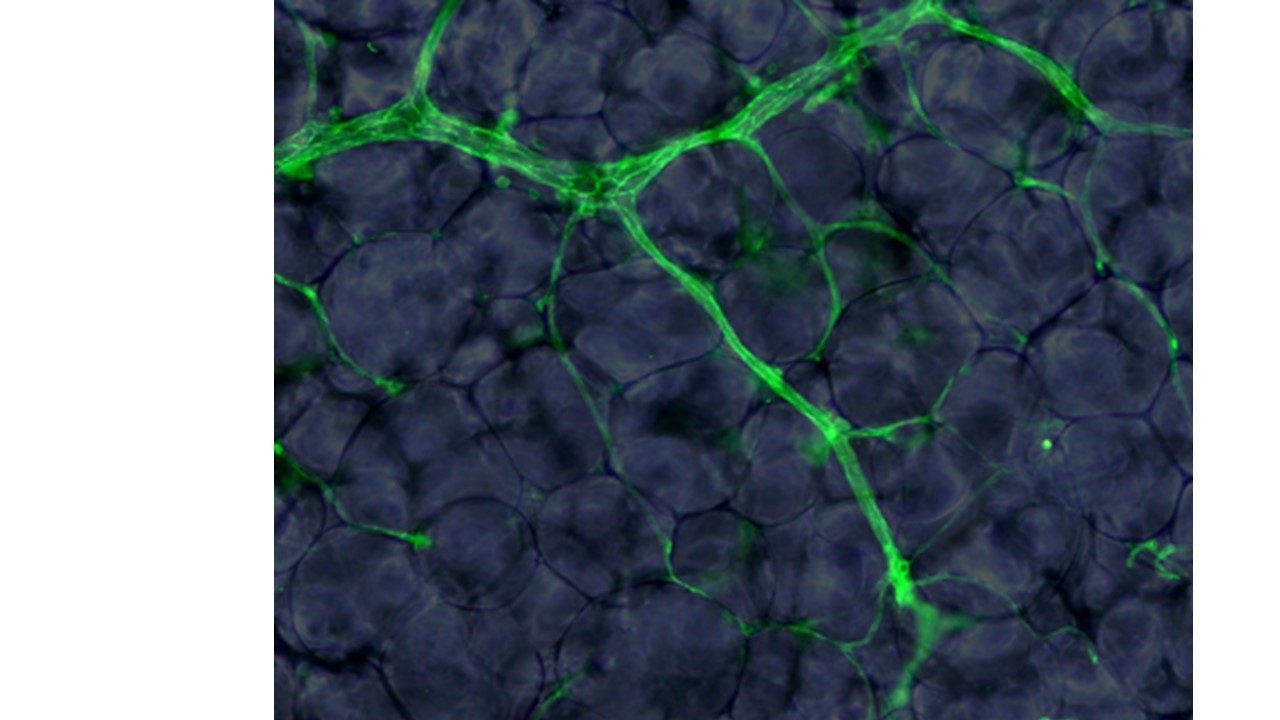
Principal investigators : Anaïs Briot, Anne Bouloumié
Our work on native and primary microvascular endothelial cells from subcutaneous and visceral human and mouse adipose depots aims at 1) identifying the cellular and molecular mechanisms involved in nutrient sensing and transport, 2) defining the inter- and intra-fat depot phenotypic and functional heterogeneity of endothelial cells and, 3) assessing the impact of obesity and aging on adipose depots endothelial cells metabolic function in human and mice.
From progenitor cells to mature adipocytes: Heterogeneity, differentiation and function
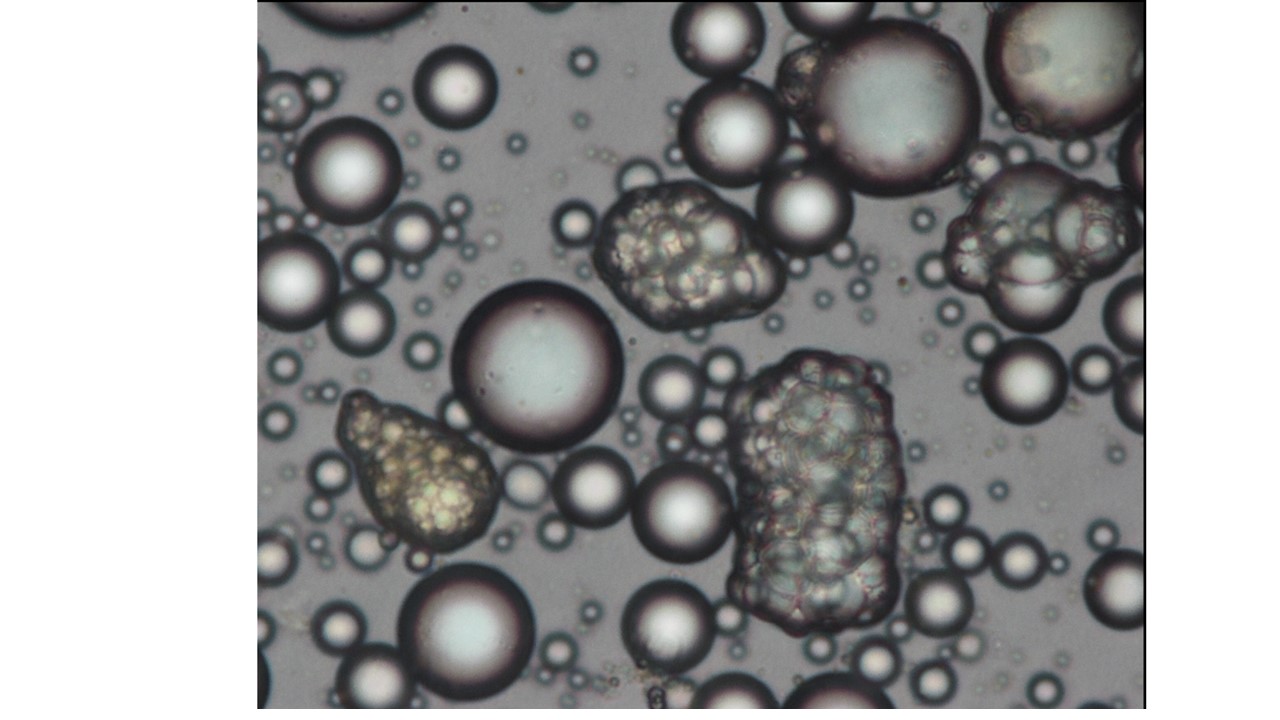
Principal investigators : Anne Bouloumié
Our objectives are to define the intrinsic and extrinsic molecular mechanisms governing the heterogeneity of adipocytes and progenitor cells in terms of their fat (white, beige and brown) and myofibrogenic fate and their intra- and inter-depot niche in physiological and pathological contexts of natural or obesity accelerated aging.
Dynamics and adaptation of adipose depot:
VASCULOMetabolic flexibility and fasting
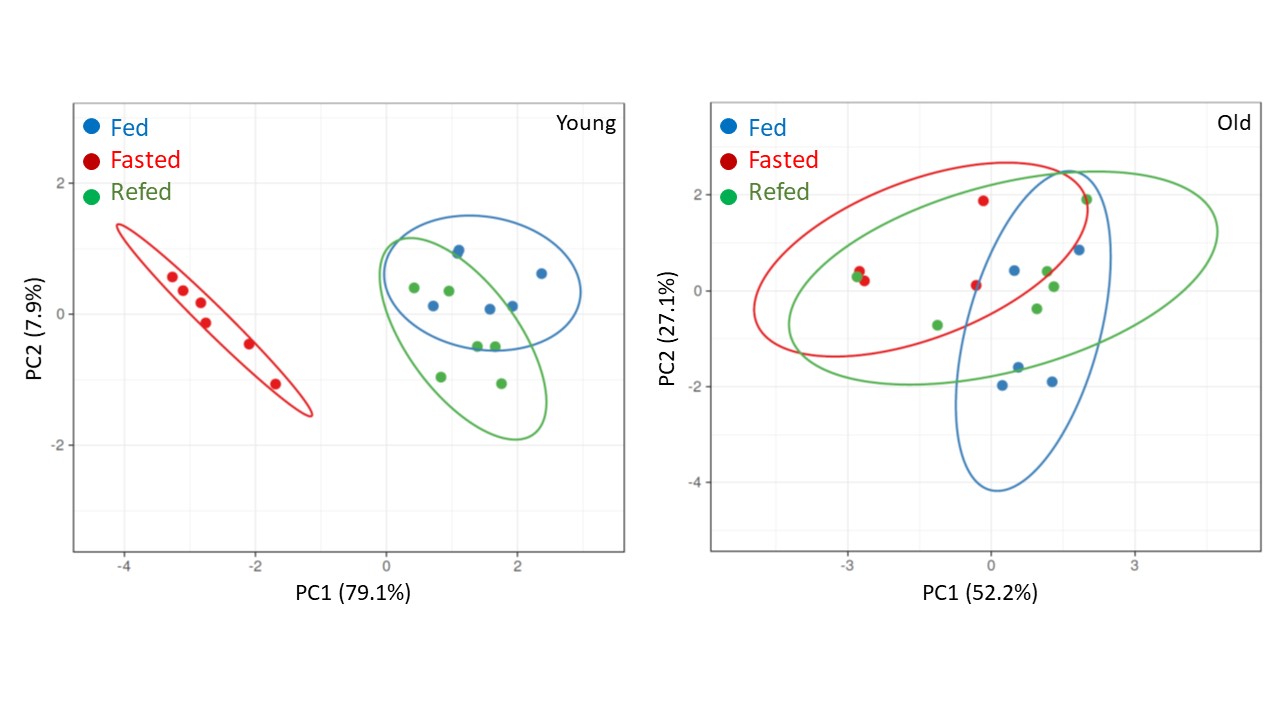
Principal investigator : Anaïs Briot
Using distinct fasting protocols, our objectives are 1) to identify dynamic combinations of markers (metabolomic, transcriptomic and metagenomic) of metabolic inflexibility, 2) to assess the beneficial and deleterious consequences on the phenotype and functionality of adipose depot cells, and 3) to identify cellular and molecular mechanisms that selectively mimic the beneficial effects of intermittent fasting according to sex, age and metabolic health.
Adaptability of lymphatic endothelium in metabolic diseases
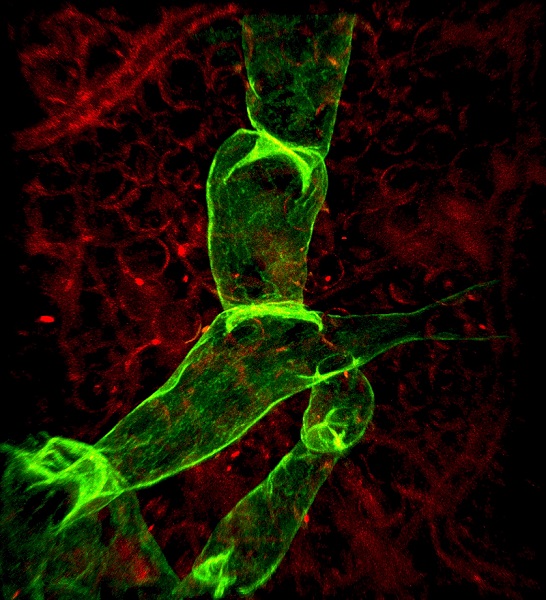
Principal investigators : Florence Tatin, Anais briot, Anne Bouloumie
Florence Tatin is interested in understanding the adaptability and heterogeneity of lymphatic endothelium in metabolic diseases. We are interested to determine the spatial organization of the lymphatic network in adipose tissue microenvironment with new approaches of 3D imaging by light sheet microscopy (IMACTIV-3D). In addition, obesity is well-recognized as an important rick factor for lymphatic dysfunction. We aim to better understand how adipose tissue may influence and interact with lymphatic endothelial cells, and inversely how dysfunction of lymphatic vessels may alter cell-responses of the adipose tissue. Towards this aim, we develop single-cell RNAseq analysis of endothelial cells, and cell sorting strategy associated with cell biology and biochemistry approaches.
Adipose deposits-intestinal epithelium dialogue & tissue alteration in obesity, inflammatory bowel Disease anD Cancer
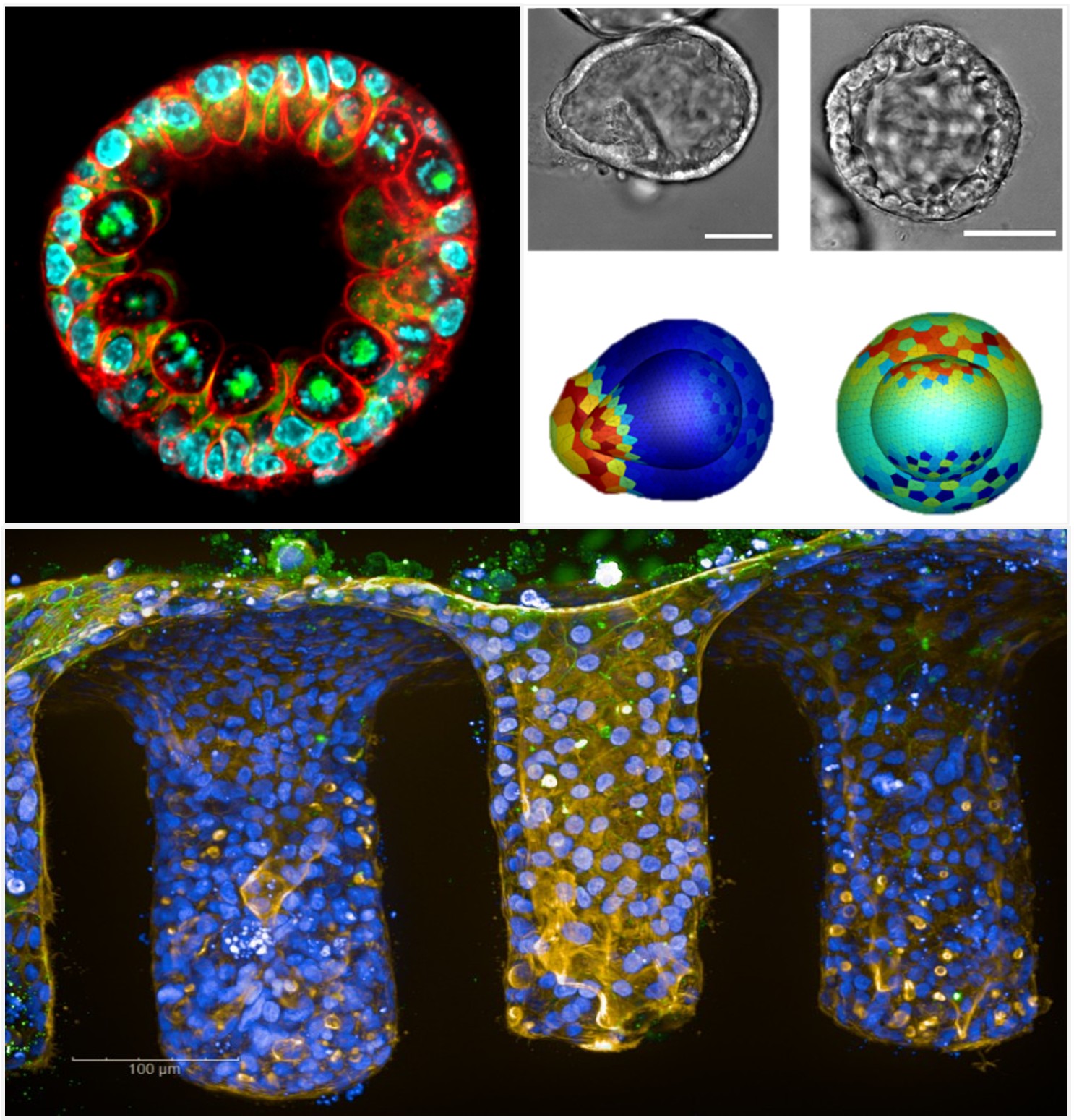
Principal investigators: Audrey Ferrand, Anne Bouloumié
We hypothesize that, in the context of obesity, architectural, metabolic, and inflammatory changes in mesenteric and omental fat deposits, as elements of the intestinal crypt macroenvironment, contribute to the dysfunction of intestinal stem cell renewal capacities and compromise the barrier function of the epithelium. Furthermore, in chronic inflammatory bowel diseases (IBD) and colorectal cancer, alterations in the epithelium and stroma could in turn influence the metabolic and immune properties of these fat deposits.
By combining functional, pharmacological, and architectural analyses with multidisciplinary approaches integrating biomechanics, bioengineering, 4D imaging, and advanced machine learning and deep learning methods, we aim to characterize the interactions between the intestinal epithelium and its micro- and macro-environment, in particular the stromal niche and adipose deposits, and thus elucidate their contribution to the pathophysiological mechanisms involved in obesity, IBD, and colorectal cancer.
These projects are being developed as part of the I2MC’s NAMs research program and the MED-OOC PEPR.(https://www.pepr-medooc.fr/).

RECENT PUBLICATIONS
Non-destructive assessment of multi-material microtissue mechanics reveals the critical role of rigidity gradients in tumour growth and pressure. Ala A, Douillet C, Ferrand A, Velay V, Segonds S, Recher G, Bugarin F. Acta Biomateriala. 2025. In press.
3D imaging and single-cell analysis reveal cellular heterogeneity of lymphatic valve endothelial cell types. E, Morin R, Iacovoni JS, Draia-Nicolau T, Gomes A, M, Bernes-Lasserre P, Lagarde JM, AC, Garmy-Susini B, Bouloumié A, Anaïs Briot A, Tatin F. iScience. 2025. Open access
Unveiling how mitotic spindle orientation in 3D human colon organoids affects matrix displacements through a 4D study using DVC. Magne L, Pottier T, Michel D, Laussu J, Bonnet D, Alric L, Segonds S, Recher G, Bugarin F, Ferrand A.Sci Rep. 2025. PMID: 40595987. Pubmed.
Lack of fibro-inflammatory response in human mammary adipose tissue in obesity. Fallone F, Rebeaud M, Bouche C, Fontaine J, Arellano C, Ducoux-Petit M, Orgerit L, Deudon R, Nicolle R, Franchet C, Estève D, Mouton-Barbosa E, Dauvillier S, Moutahir M, Burlet-Schiltz O, Bouloumié A, Vaysse C, Muller C. Int J Obes (Lond). 2025. PMID: 39738492. Pubmed.
Deciphering the interplay between biology and physics with a finite element method-implemented vertex organoid model : A tool for the mechanical analysis of cell behavior on a spherical organoid shell. Laussu J, Michel D, Magne L, Segonds S, Marguet S, Hamel D, Quaranta-Nicaise M, Barreau F, Mas E, Velay V, Bugarin F, Ferrand A. PLoS Comput Biol. 2025. PMID: 39792958. Pubmed.
Endothelial DLL4 is an Adipose Depot-Specific fasting sensor regulating fatty acid fluxes. Aupetit A, Decaunes P, Belles C, Riant E, Galitzky J, Chapouly C, Laisné M, Flores-Flores R, Chaput B, Vié K, Arnal JF, Bouloumié A, Briot A. Arterioscler Thromb Vasc Biol. 2023 PMID: 36924232. Pubmed
Notch activation shifts the fate decision of senescent progenitors toward myofibrogenesis in human adipose tissue. Boulet N, Briot A, Jargaud V, Estève D, Rémaury A, Belles C, Viana P, Fontaine J, Murphy L, Déon C, Guillemot M, Pech C, Veeranagouda Y, Didier M, Decaunes P, Mouisel E, Carpéné C, Iacovoni JS, Zakaroff-Girard A, Grolleau JL, Galitzky J, Ledoux S, Guillemot JC, Bouloumié A. Aging Cell. 2023 Jan 8:e13776. Pubmed
Periprostatic Adipose Tissue Displays a Chronic Hypoxic State that Limits Its Expandability. Am J Pathol. 2022 Jun;192(6):926-942. Pubmed


Inserm/UPS UMR 1297 - I2MC Institut des Maladies Métaboliques et Cardiovasculaires
1 avenue Jean Poulhès - BP 84225 - 31432 Toulouse Cedex 4
Tél. : 05 61 32 56 00
Horaires
Du lundi au vendredi
8h30 - 12h30 / 13h45 -16h45












